peyer's patches are found in the distal portion of the
 Peyer's patch - Wikipedia
Peyer's patch - WikipediaPeyer (PPs) patches refer to organized lymphoid aggregates located within small and large intestines of many mammal species (Liebler-Tenorio and Pabst, 2006). From: Related Terms: Mark Coles, ... Henrique Veiga-Fernandes, in , 2010E Requirements for the development of the Peyer patches The PPs are specialized SLOs located in the lamina proria of the mucosa and extending in the submucosa of the ileum. Adult PPs have a similar structure and organization such as NL, with B and T cells segregated in well-defined and organized regions. 87 Curiously, this pattern of lymphocyte segregation B and T is totally independent of the infection or stimulation of antigens, since germ-free mice also show discrete areas B and T cells 88 However, there are specialized aspects for the organization of mature PPs. Thus, the luminal side of the PP exhibits a protrusion that is covered by an enthermal epithelium layer associated with follicles that contains specialized epithelial cells, designated as M cells, which play an important role in the mediation of immune functions. 89 Compared to NLs, PPs are integrated with the lymphatic network in a slightly different way as they lack aferent lymphatic. However, PPs have eferent lymphatic that emerge from the lymphatic sinuses on the side of the PP serosal and drain the lymphatic and immune cells into the mesentric NL and then into the thoracic duct.90In mice, PP development occurs during embryonic life. PPs are developed in variable numbers (5 to 12) on the antimenenteric side of the mediaintestine, initiating their development in the duodenum and the proximal ileum; later, the most distal primeval PP is formed at more or less regular intervals in the lower part of the medium-gut.54,91 Keiichiro Suzuki, Sidonia Fagarasan, in 2010 The development of PPs was recently widely revised (Eberl and Lochner, 2009; Finke and Meier, 2006). It is enough to say here that PPs develop from E15.5 and their formation requires multiple interactions between the hematopoietic RORγt+LTi cells and the estromal cells that involve the LT-LTβR axis, few key cytokines, and the chemokines and their receptors (i.e., IL-7/TSLP-IL-7R, CXshi5 et Fink) All these complex interactions lead to the recruitment of the PP lymphocyte anagen that will be segregated in cell phones B and T cell area. PPs are present in germ-free mice, although their size is small. However, after bacterial colonization of the intestine, the size and composition of PP changes, and the vast majority of PP lymphocytes become active cells that form germ centers (GCs; Macpherson, 2006). Avi N Kogan, Ulrich H von Andrian, in , 200810.3.2 Patches de Peyer10.3.2.1 Anatomy Peyer's patches (PPs) are a series of small lymphoid organs located on the wall of the small intestine. Above the prominent follicles of B cells and the interfollicular areas of T cells is the most diffuse subbepithelial dome (SED); all of them are enclosed by an epithelium associated with follicles (FAE) that forms the boundary between PP and the intestinal lumen (revised in Refs 182-184). PP is the first line of defense of the immune system against mucous antigens associated with the intestine. This is largely due to the specialised epithelial cells of the FAE called M cells, which can take the antigen directly from the intestine and channel it through the vesicles through the epithelial layer (revised in Refs [183,185]). Although it is still possible for M cells, despite their lack of class II MHC expression, to be able to some degree of antigen processing, it seems more likely to pass along the antigen to APC for this task [184]. Curiously, the pockets rich in B and T that are immediately below the M cells are only sparsely populated by APC capable of providing strong cost-effective support [183,185]. However, APC as DC and macrophages are abundant in the adjacent SED, where they come in close contact with lymphocytes.10.3.2.2 Microvascular specialization Post-capilar venules in PP also have HEV morphology, but while PP HEV resembles PLN HEV in appearance, its expression profiles of traffic molecules are quite different. PP HEV expresses PNAd on its abluminal but non-luminal surface [158]; however, they express high luminal amounts of the mucosal direction MAdCAM-1. Unlike PLN, PPs also contain several venular trees, which originate in areas associated with PP B cells but are more closely associated with T-cell interfollicular areas. PP HEV can be subdivided into follicular (i.e., B cell foicle-asociated) and interfollicular (T-cell-asociated area) HEV. Of these two subcategories, the interfollicular HEV is greater in number. PPs do not have associated aferent lymphatic; the antigen comes mainly through M cellular transport, although DC extensions have also been reported to penetrate the intestinal epithelia and the antigen of the sample directly [186]. Different lymphatics leave the PP; however, by directing cells and possibly soluble antigen to the mesenteric lymph nodes (MLN) placed downward (Section 10.3.4).10.3.2.3 The migration of lymphocytes to PPLike PLN, PPs recruit lymphocytes in large numbers, but the adhesion cascades used in these two SLOs differ in significant ways. Naïve The T-cell bearing in PP HEV is initiated by both L-selectine and α4β7 integralino, which mediate the behavior of different lymphocytes bearing. Most tethering is initiated by L-selectine, but α4β7 plays a key role in reducing the speed of rolling lymphocytes, while also mediating some initial interactions of HEV lymphocytes [187-189]. These latter features make α4β7 the adhesion molecule associated with predominant PP, at least in mice; in the PP homage, α4β7 is much more capable of compensating the absence of L-selectine than vice versa [190,191]. Both L-selectine and α4β7 are joined by MAdCAM-1, which is unique in its function as ligand for selectines and integrators [187–189,192]. While α4β7 may mediate the bearing before activation, it can only initiate firm detention in its activated state [193]. The activation signal for α4β7 and LFA-1, both of which mediate the firm adherence of T ingenuous cells in PP HEV, is given by CCL21 and/or CCL19 [189,194]. The α4β1 fundamentalist has also been found to play a minor role in the tribute to PP [195]. It is believed that central memory T cells use the same molecular pathways in the PP homing as ingenuous T cells, although the roles for additional chemokines and other adhesion molecules have not been formally excluded. Like T cells, B cells also use L-selectin and α4β7 to migrate to PPs. The migration of B-cells to PP is similar to the concentration of B-cells to PLN in which the ligands for CXCR4 and CCR7 are involved in the activation of the integraine; in PP, however, CXCR5 plays an equally prominent role. CXCR5 is expressed in all peripheral B cells, as well as in the TFH subset of CD4+ memory T cells (Section 10.1.2.1). Studies in mice have shown that cellular B-to-PP homage is facilitated by both CXCR5- and by signaling pathways interrupted by CXCR4/CCR7, with each track capable of partially compensating the absence of the other [168]. Curiously, it has been reported that T and B cells are subject to firm arrest in different regions of the PP venular tree. Intravital microscopic studies in mice have revealed that T cells preferably stop in the interfollicular HEV of CCL21hi, while B cells arrest more often in the FL21low/. This is suggestive of the PP HEV specialization at the chemokine presentation level and possibly production, most likely implies the additional location of the ligand CXCL13 CXCR5 in follicular HEV. On the contrary, PLN B cells must first emigrate through T-cell areas to reach the B.10.3.2.4 DC follicles Migration to PPDC and its precursors cannot reach PP through aferent lymphatic, as mentioned above, there are no lymphatics. PP DC must originate by default from DC or DC precursors circulating in the blood, probably entering through HEV. PP DC finds and processes the antigen within the subepithelial dome (SED), where they are well positioned to collect the antigen coming through CCR6 cells and their ligand CCL20 have been involved in the DC location within the murine PP, such as CCR6+CD11b+ DC locates the SED and CCR6−CD8α+ DC to the interfollicular regions. PP CD8α+ DC expresses CCR7 instead and migrates to CCL21 and/or CCL19 produced in the areas of T-cell. A third party, CD11b−CD8α− PP DC also expresses CCR7, but is found both in the SED and in the IFR [196]. More recently, it has been suggested that CCL9 attracts CD11b+ DC to the SED [197], perhaps regardless of CCL20/CCR6, although the DC counter-receptor is unknown.10.3.2.5 PPLymphocytes PP lymphatic graduate through efferent lymphatics, which channel them to the MLN (Section 10.3.3). IAPA1 released by lymphocytes provides crucial signals for lymphocyte egress here as elsewhere (Section 10.1.2.5). Kenneth R. Youngman, Eugene C. Butcher, in , 2005 Chemokines in the lymphocyte homage to Peyer's patches Peyer's patches are the frontline lymphoid organs of the intestinal tract. Here, the antigens of the intestinal lumen are collected by the M cells of the epithelium of the dome, where it is thought that the dendrite cells, recruited in part by the local production of the epithelial chemoquina MIP1-α/CCL20, process the antigen for the presentation to the naive memory and lymphocytes that recirculate through the organ (allwasaki and Kelsaki). Ingenuous lymphocytes and subsets of α4β7+ memory recirculate through PP HEVs. Although B and T cells share the use of L-selectine and α4β7 in initial contact and in slow rolling, and LFA-1 in detention, the T and B detention sites of cell signature are segregated within PPs (Warnock et al., 2000). The HEVs that support the accumulation of B cells are concentrated in the follicles or near them, the objective domain of most B cells that enter PP, while T cells accumulate preferably in interfollicular HEV (Fig. 34.4). The SLC/CCL21 of the chemoquina expressed by HEV T cell arrest on HEV in the T cell zone through CCR7 on the T cell surface. Instead, although B cells can also use CCR7, many B cells are activated to bind HEV segments up from the T HEV area into segments of vessels within or adjacent to B cell follicles (Fig. 34.4). In this case, arrest may be triggered by any of several chemotherapys, including SDF-1/CXCL12 and BLC/CXCL13, as well as SLC/CCL21 (Okada et al., 2002). Fig. 34.4. Lymphocyte location in PP-HEV. Immunohistology of subpopulations of lymphocytes accumulated in PPs. Left, T-cells accumulated in HEV SLC+ interfollicular segments; media B-cells in HEV SLClow segments associated with primary follicles; right, segregation of Preferential T-cell and B-cell accumulation sites in relation to follicles. TRITC-labeled T cells (left) or B (medium and right) and T cells labeled by CMFDA (right) were observed in normal animals' HEVs. This unexpected level of specialization of HEV can allow differential and segmental control of the subset of lymphoid microenvironment lymphoid lymphoids functionally different in vivo, especially in PPs. John J. Cebra, ... Helena Tlaskalova-Hogenova, in , 2005Peyer's patches (PP) and solitary follicles (SF)Peter's patches (about 8-10) and SF are found on the walls of the small intestine or small and large intestines, respectively. About 30,000 SF occur in the human intestine, and about 10–20 SF occur in the small murine intestine, as described recently (Brandtzaeg and Farstad, 1999; Hamada et al., 2002). These organized lymphoid structures are composed conspicuously or primarily of spherical lymphoid follicles B, including chronically present B lymphoblates embedded in a follicular dendritic cell mesh (DC). GCR cells follicles are secondary reactions and show chronic reactions from the germinal center (Weinstein et al., 1991; Liu et al., 1991). The predominant IgM isotype expressed about GC B explosions in PP and SF of most mammal species is IgA; B blasts in human tonsillar GC mainly express IgG isotypes (Lebman et al., 1987; Pascuel et al., 1994). Both tissues normally contain a relatively large number of B IgA (Peyer patches) or IgG (tonsils) "memory" cells of B cells. Thus, the B-cell follicles of these mucous tissues differ from those of the spleen and lymph nodes, which are generally quiescent and "primary", showing no GCR with B-divisor blasts and which are composed of positive IgM/IgD primary B cells. PP, SF, and also murine NALT cell follicles contain microplete (M), disseminated through their epithelium associated with follicles (FAE). These M cells act as "efferent policies", transporting samples of foreign antigen from the intestinal lumen or respiratory tract to the organized lymphoid tissue (Weltzin et al., 1989; Hamada et al., 2002; Zuercher and Cebra, 2002; Zuercher et al., 2002). Although the SF of mice shares many PP characteristics and differs from "cryptopatches" (see Kanamori et al., 1996), it also seems to contain some of the cells that are characteristic of cryptoparches, especially c-kit+, IL-7R+, Tu1+ cells involved in the lymphopois of some T intestinal cells (Hamada et al., 2002). Most T lymphocytes in PP reside in the interfollicular regions in the form of a wedge and include CD4+ and CD8+T cells (London et al., 1990). Both subsets generally include a higher proportion of cells that show activated phenotypes, such as CD45RBlow, CD69+ and CD62Llow (Talham et al., 1999; Jump and Levine, 2002), which corresponding subsets of quiescent spleen or lymph nodes. Recently, the in vitro culture of PP T cells on anti-CD3 plates has shown that a "naturally active" population of CD45RBlow, CD4+ T cells can be stimulated through TCR to produce much larger amounts of IL-10, but not IFNγ, IL-4, or IL-12, compared to MLN and PLN (Jump and Levine, 2002). Thus, PP appears to contain a set of T cells that express properties similar to those that can mediate oral tolerance or deregulation of peripheral T cells. It has been shown that CNV (CD11c+ DC) interdicted PP-interdicted DC selectively induces the production of IL-4 and IL-10 by naïve CD4+ T-cells of TCR transgenic mice (Iwasaki and Kelsall, 1999). Such DCs are also found in the intestinal LP and MLN (see the section on Gut Lamina Propria). Recently, Iwasaki and Kelsall (2001) have distinguished three DC subsets in PPs. The DCs CD11b+/CD8α (myloid), located in the sub-bepithelial dome, appear to be the subset that produces IL-10 by stimulating with the CD40-ligand trimer and which may present Ag-peptide to stimulate CD4+ Cells T to produce IL-4 and IL-10. Thomas T. MacDonald, Giovanni Monteleone, in , 2005IMMUNE FUNCTION IN HUMAN GALT The PP is clearly the site for the induction of the secret-IgA response (S-IgA) (Chapter 21). However, there has been very little functional work on T cells in the human GALT. Several years ago, we observed that it was possible to visualize PP in the ileums of children subjected to colonoscopy on suspicion of inflammatory bowel disease (MacDonald et al. 1987). PP could be biopsy selectively and compared to biopsies taken from adjacent mucosa. Thus it was possible to test both the inductive phases and the effects of the human mucous immune response. If a comparison is made of the spontaneous production of cytokines by enzymatic immunospot (ELISPOT) of peripheral T cells, PP cells and T cells of mucosa lamina of normal children, several eye-catching characteristics emerge (Hauer et al. 1998). As expected, blood cells do not show virtually secret cytokine cells. The PP, however, shows a very high percentage of cells that continue to secrete cytokines when taken from the patient and cultivated during the night. The answer is dominated by the interferon-γ-secretariat cells, with few interleukin-4-secretariat cells (IL-4-secretariat), IL-5-secretariat, or IL-10-secretariat (Fig. 22.4). The same pattern is seen with T-prone lamina cells, and if anything, the interferon-γ response is greater. When PP cells are placed in culture, they spontaneously release large amounts of interferon-γ (IFN-γ) into the supernatant of culture and only trace amounts of IL-2, IL-4, IL-5, or IL-10 (Monteleone et al. 2003). Fig. 22.4. When Peyer's normal cells are placed in culture at night, there is spontaneous production of IFN-γ and insignificant production of IL-2, IL-4, IL-5, or TNF-α. To emphasize this, ELISPOT data on Peyer's normal human patch cells are also shown. The IFN-γ ELISPOT well contained 5000 cells, while the IL-4 well contained 40,000 cells, and yet it is clear that the response is dominated by IFN-γ. In an effort to determine whether specific antigen-specific Th1-type responses were also produced, PP cells were stimulated with in vitro cow-milk-milk antigens in an effort to generate a memory response (Nagata et al. 2000). Surprisingly, PP T cells gave a specific strong response to the antigen to β-lactoglobulin (β-lg), while T cells of peripheral blood gave weak responses. The response was verified by the observation that β-lg caused a large increase in the number of CD4+, CD25+ in PP cultures, but not in peripheral blood. These results were also verified by polymerase chain reaction (PCR). In seeking an explanation for this response, it was observed that PP contained abundant transcripts for IL-12 p40, that PP cells secreted IL-12 p70 in cultural supernatants, and that IL-12 p70 could be seen in cells under the dome epithelium. The abundance of IL-12 in the PP means, therefore, that when a T cell responds to a nominal antigen of peptides, it does so in a sesgado ambience towards Th1, which explains the high level of spontaneous cells betweenferon-γ-secretariat and the answer dominated by interferon-γ to β-lg. By western swelling, IL-12 p70 was easily demonstrated in human PP protein extracts. The T-cell response to IL-12 depends on the expression of the high affinity IL-12 receptor (IL-12R) composed of two subunits, IL-12Rβ1 and IL-12Rβ2, with the IL-12Rβ2 chain being the critical signaling component. IL-12Rβ2 RNA was systematically detected in CD4+ and CD8+ PP lymphocytes and lamina propria (LPL) but not peripheral blood cells (PBL) (Monteleone et al., 2003). The effect of the IL-12 on the Th1 cell differentiation depends specifically on the fast phosphorylation and activation of the signal transducer and transcription activator 4 (STAT4). STAT4 activated was seen in all PP biopsies and mucosa analyzed, but not in newly isolated human PBL. On the contrary, the active STAT6, a transcription factor induced by IL-4 and associated with the development of Th2 cells, was expressed weakly in PP and ileal mucous biopsies. STAT4 was detectable in the nuclear extracts of PP T cells and the EMSA analysis demonstrated the specific STAT4 binding to target DNA. The new transcription factor, T-bet, is enough to start the Th1 differentiation and repress the Th2 cell responses. Nuclear proteins extracted from PPL show immunoreactivity with anti-T-bet (Monteleone et al., 2003). Antibody anti-IL-12 almost completely inhibited the increase in interferon-γ production induced by superantigen PP cells. In general, therefore, the evidence is quite overwhelming that the default T-cell response in the human terminal ileum PP is along the Th1 track. In 1998 the Peyer patches Peyer's patches are located in the intestine. With regard to function and morphology, they are analogous to tonsils. However, there are also numerous solitary lymphoid nodules scattered along the lamina of the intestine. Its main function is the production of immunoglobulin A (IgA) along with other types of immunoglobulins. Epithelial cells that cover areas of Peyer patches can be identified as M cells with micropinocytic properties, allowing cells to show antigens. Under this specialized epithelium area there are additions of T cells, B cells and plasma cells. The lymphocytes that house the Peyer patches show an adhesion molecule called VLA-4 (very late antigen) (Gershwin et al., 1995). Hiromichi Ishikawa, ... Hiroshi Kiyono, in , 2005UNIQUE CHARACTERISTICS OF GALTPeyer's patches (PP), the appendix, and solo lymphoid nodules, the main components of GALT, serve as the mucosal inductive sites for the GI tract, and the tonsils and adenoids, collectively identified as NALT, do the same for the upper respiratory tract 2001 In addition, a recent study has provided direct evidence that isolated lymphoid follicles (ILF) are equipped with immunological characteristics that are in some aspects similar to those of PP (Hamada et al. 2002) and should therefore be considered as part of the GALT (see text below). Of all GALTs, the murine PP has been the most extensively characterized as mucous inductive tissue (Iijima et al. 2001; Mowat 2003). The PP surface is covered by a unique epithelial layer known as the epithelium associated with follicles (FAE). The FAE is enriched with specialized antigen cells that are known as microcarpet cells (M), as well as called due to the irregular and shortened microvilli that characterize them only (Owen and Jones 1974). On the site of the basal membrane, M cells form an apparent pocket, containing T cells, B cells, macrophages and DCs. M cells, characterized by small cytoplasmic vesicles and few lisosomes, are adepts of intake and the transport of luminal antigens (Owen et al. 1988). It is believed that the absorption of antigen by M cells does not result in the degradation of the antigen, but in the delivery of the antigen intact to the underlying APC (Owen and Bhalla 1983). In addition to the transport of luminal antigens, M cells serve as input port for pathogens. For example, the invasive strains of Salmonella and reovirus begin the infection by invading the M cells of PP (Wolf et al. 1981; Weinstein et al. 1998). Therefore, M cells can be regarded as a gateway to the mucosal immune system (Neutra et al. 1996a). Anatomical, the PP is characterized by a dome configuration, with the area just below the known FAE to enrich with B cells controlled by IgA, Th1 and Th2, macrophages and DCs, everything necessary for the induction of immune responses specific to the antigen. After absorption and delivery by M cells, antigens are processed and presented immediately by the DCs as APCs (Kelsall and Stober 1996). At least three DC subpopulations have been described in PP: myeloid (CD11b+), lymphoids (CD8α+), and double negative (Iwasaki and Kelsall 2000, 2001) (see Chapter 26). Myeloid DCs in the subepithelial dome (SED) lacks ripening markers such as DEC-205 and therefore appear to be immature. The lymphoid and double-negative DCs induce the Th1 differentiation necessary for the subsequent development of the immune mediated by cells (CMI), and the myeloid DCs induce the required Th2 cells for the generation of IgA immune responses in the mucous effects sites. The lymphoid and double-negative DCs are classified as belonging to the DC1 subtype, and the myeloid DCs belong to the DC2 subtype (Banchereau et al. 2000). After exposure to safe food antigens, myeloid DCs produce IL-10 and TGF-β and can contribute to the induction of systemic inresponsibility to the orally administered antigen as oral tolerance or mucous tolerance by mediating the differentiation of Th3 or T regulator (Tr) cells (Iwasaki and Kelsall 1999, 2000). The myeloid DCs in the SED region express the CCR6 chemokin receptor, whose ligand, CCL20, is expressed by the FAE. Mature myeloid DCs express improved levels of the CCR7 chemokin receptor, which migrates from SED to the interfollicular region (IFR), where it interacts with T cells (see chapters 26 and 34). Different follicles (cell B zones), which contain germinal centers for the division of B cells, are located directly below the FAE region. Because these PP germ centers are considered the site of affinity maturation and frequent IgM-cell isotype switches to IgA (Sonoda et al. 1989), it is believed that they contain most positive IgA-surface (sIgA+) Cells B. Adyacente to the B-cell zones are found to be areas dependent on T-cells, but mature T-cells. About 65% of αβ T cells are CD4+ CD8 and exhibit Th cell properties. After the presentation of the antigen by the relevant DCs described in the previous text, these Th cells differentiate in the Th1 and Th2 type cells necessary for the induction and regulation of the specific antigen CMI and IgA responses, respectively. About 30% of αβ T cells are CD4− CD8+ and contain CTL precursors (London et al. 1987). Thus, organized inductive tissues (e.g. PP) are equipped with all immunocompetent cells necessary for the initiation of antigen-specific mucous immune responses. Tapan R. Shah, Ambikanandan Misra, in , 20118.4.3.1.7 M (Microfold) Cells Peyer's patches are clusters of subepithelial follicles and lymphoids found in the intestine. They are oval or rectangular in shape and are found on the antimenomeric wall of the intestine. They are more prominent in the ileo and are characterized by specialized epithelial cells called M cells. M cells are compactly arranged with adjacent absorptive cells and create incavations that involve lymphocytes and other similar defensive components. Along with these, the bodies of Golgi, endoplasmic reticulum, mitochondria, etc. are present. These cells share close bonds with adjacent absorptive cells. They also play a key role in the initiation and triggering of the immune response. Prosper N. Boyaka, Kohtaro Fujihashi, in , 2019Customeral lymphatic tessos Each PP contains a dome region that is placed under the FAE. This dome region is populated by T cells, B cells, macrophages (MØs) and dendrite cells (DCs). It includes follicles containing germ centers. The presence of the three main types of APC in the dome (i.e., memory B cells, MØs and DCs) makes it likely that the intake of antigen will occur immediately after the release of M cells (Fig. 20.4A). M cell pockets in PP contain approximately equal numbers of T and B cells, but less MØs. About 75% of T cells are Th cells. GALT B cell follicles are enriched in IgA-bearing B cells,8 suggesting that they are major sites for B μ-α-cell commutation (chapter 4 and 7). The interfollicular regions of PP contain high endothelial venules (HEVs) (chapter 2, 11). CD4 and CD8 TCRαβ T cells are found in these interfollicular regions, with CD4 T cells that represent the predominant phenotype. Both naive T cells and memory are present in PPs, with a third in cell cycle (see Fig. 20.4; Table 20.1). Lymphooxine-α (LT-α), lymphooxine-β (LT-β), and TNF-α (Chapter 9) are critical for organogenesis of lymphoid tissue (chapter 2). LT-α−/− mice are deficient in the secondary lymph nodes,9.10, while LT-β-/− mice have mesenteric and cervical lymph nodes but lack peripheral lymph nodes and PP. Tumor necrosis factor–receptor I (TNF-RI)––– lack of mice or abnormal PP structures, while TNF-α−/– mice exhibit normal patches. Recommended Publications: We use cookies to help provide and improve our personalized service and content and ads. By continuing to accept . Copyright © 2021 Elsevier B.V. or its licensors or collaborators. Direct Science ® is a registered trademark of Elsevier B.V.ScienceDirect ® is a registered trademark of Elsevier B.V.

Peyer's Patches

Untitled Document

Untitled Document

Gastrointestinal tract 4: anatomy and role of the jejunum and ileum | Nursing Times

Lymphatic System: Overview - ppt video online download

Untitled Document

Untitled Document
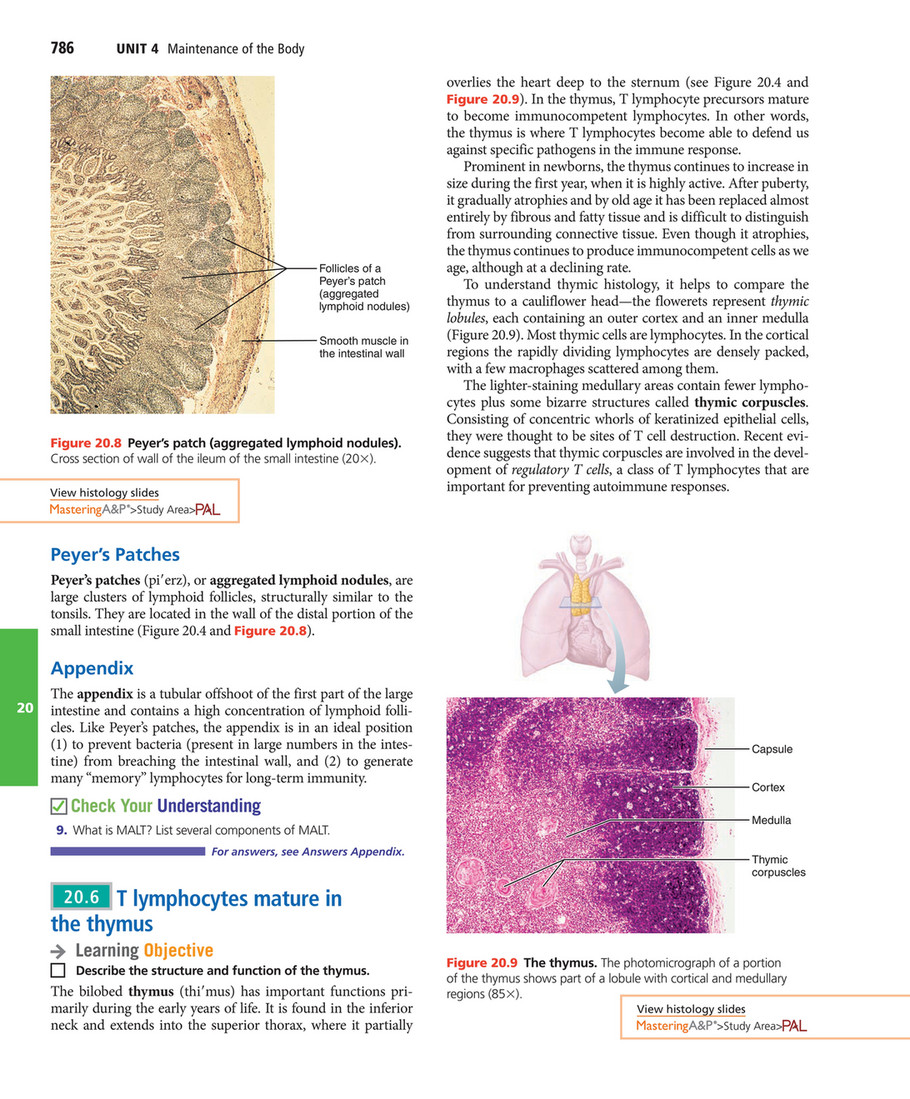
My publications - Elaine N. Marieb, Katja N. Hoehn - Human Anatomy & Physiology (2015, Pearson) - Page 796-797 - Created with Publitas.com

Lymphoid organs and system. lymphoid tissues (Primary lymphoid organs) Thymus, B.M., (in embryonic course comprise Yolk sac, Liver and spleen) lymphoid. - ppt download
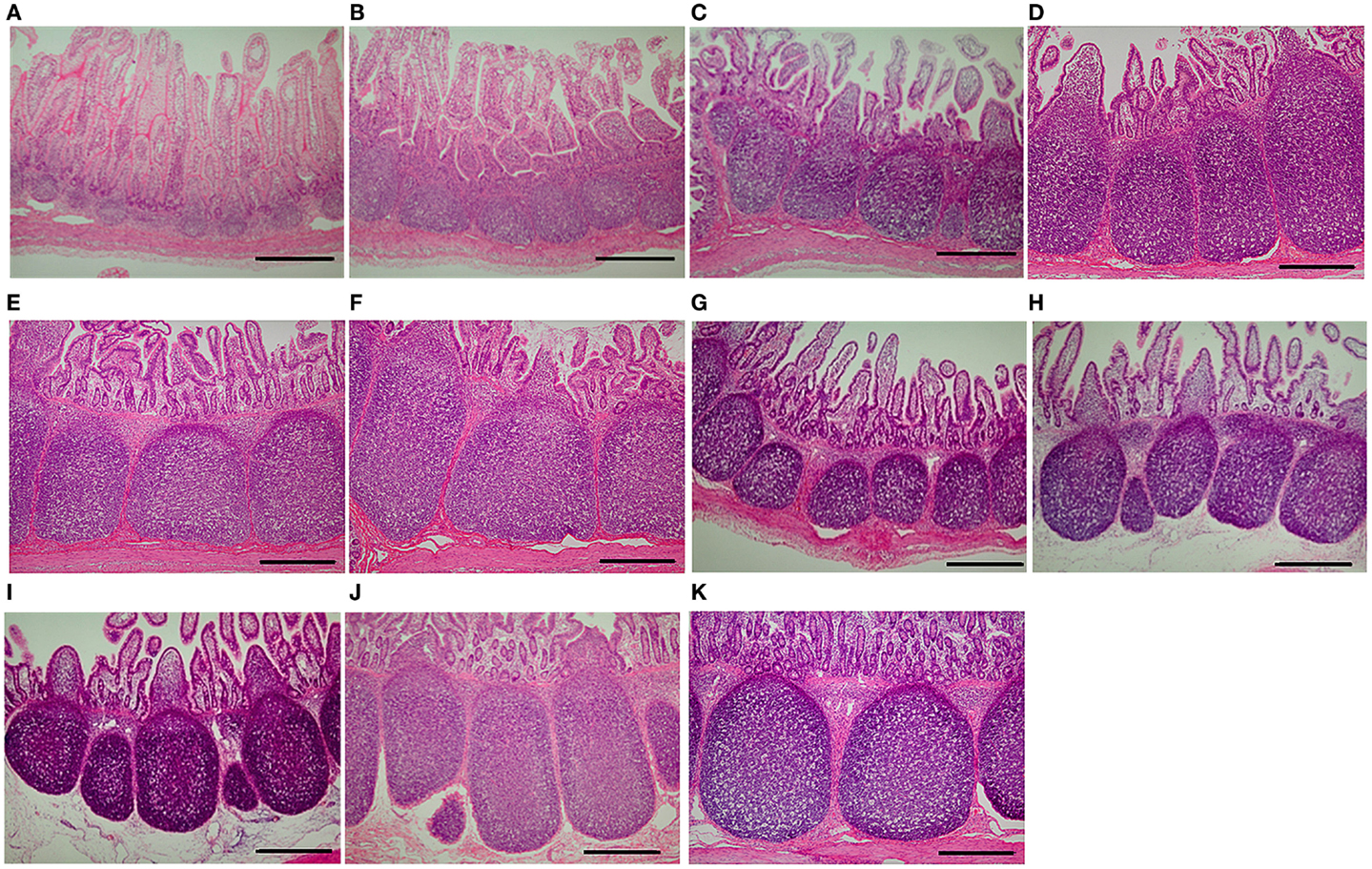
Frontiers | Weaning Markedly Affects Transcriptome Profiles and Peyer's Patch Development in Piglet Ileum | Immunology

Aggregates of lymphoid tissue present in the distal portion of sm
20 The Lymphatic System. - ppt download

Peyer's Patch - an overview | ScienceDirect Topics

Solved: 9. Select The Correct Statement About Lymphocytes.... | Chegg.com

Mastering A&P II Chapter 20 - The Lymphatic System and Lymphoid Organs and Tissues Diagram | Quizlet

The Lymphatic System. - ppt video online download

The lymphatic system 3: its role in the immune system | Nursing Times

Morphologic Observation of Mucosa‐Associated Lymphoid Tissue in the Large Intestine of Bactrian Camels (Camelus bactrianus) - ZhaXi - 2014 - The Anatomical Record - Wiley Online Library

slides.show
Peyer's Patches: Definition, Location, Function, Related Conditions

Lecture Histology Dr.Widad.J.H. - ppt download

Lymphatic System. - ppt download

Distribution of Peyer's patches in the distal ileum - Van Kruiningen - 2002 - Inflammatory Bowel Diseases - Wiley Online Library

Peyer's Patch - an overview | ScienceDirect Topics
The Lymphatic System and Lymphoid Organs and Tissues - ppt video online download

Figure 4 from Age-related changes in the anatomical characteristics of Peyer's patches in small intestine of Bactrian camels (Camelus bactrianus) | Semantic Scholar

The Lymphatic System Anatomy & Physiology. - ppt download
Thymus Thymus is the site of T-Cell differentiation and maturation it is a biolobed gland, situated above heart in the thorax region each lobe is encapsulated. - ppt download

The lymphatic system 3: its role in the immune system | Nursing Times
A Perspective Of Intestinal Immune-Microbiome Interactions In Alcohol-Associated Liver Disease
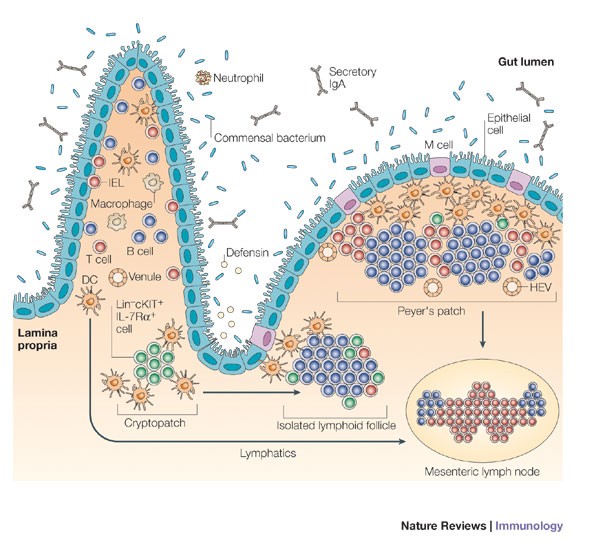
Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? | Nature Reviews Immunology

Small organs associated with lymphatic vessels are termed a Lymph follicles b | Course Hero

Aggregates of lymphoid tissue present in the distal portion of sm
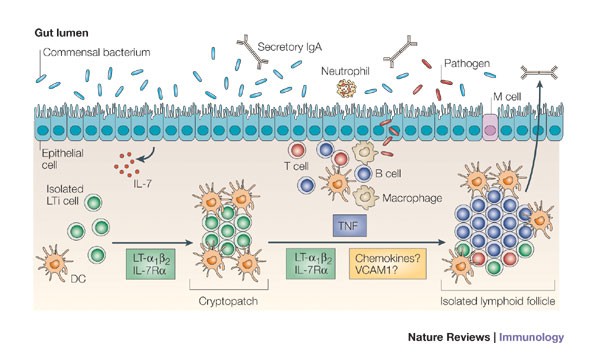
Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? | Nature Reviews Immunology

Large intestine - Wikipedia

Duodenojejunal Flexure - an overview | ScienceDirect Topics
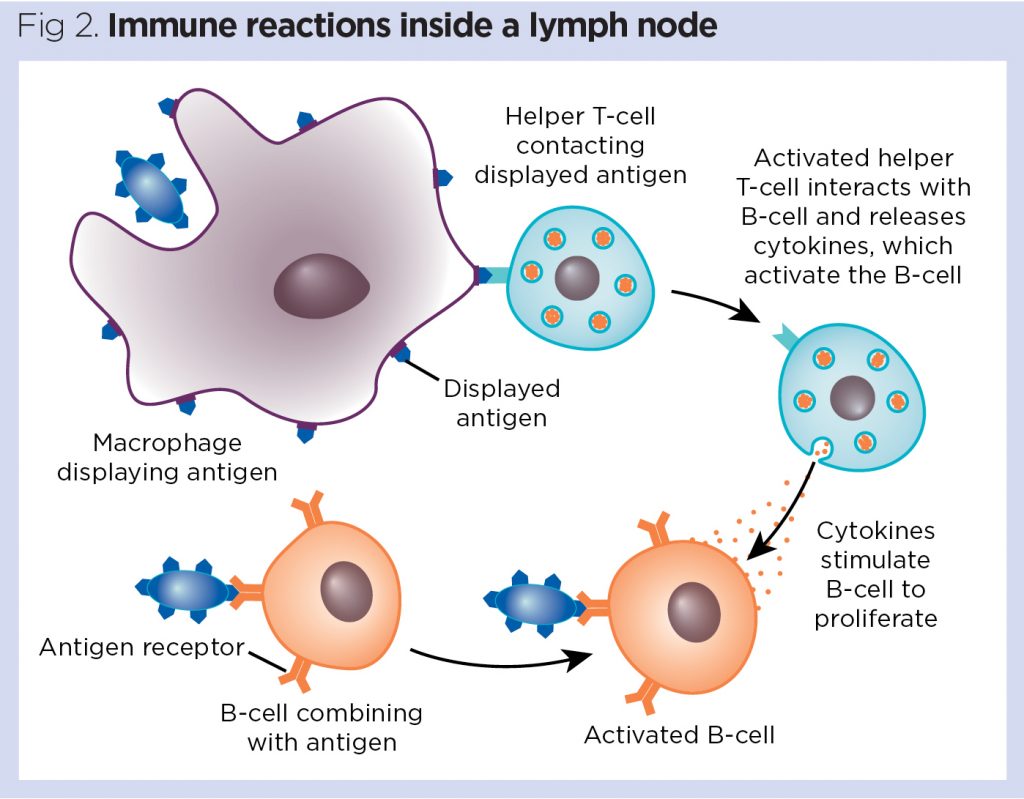
The lymphatic system 3: its role in the immune system | Nursing Times

Mastering A&P II Chapter 20 - The Lymphatic System and Lymphoid Organs and Tissues Diagram | Quizlet
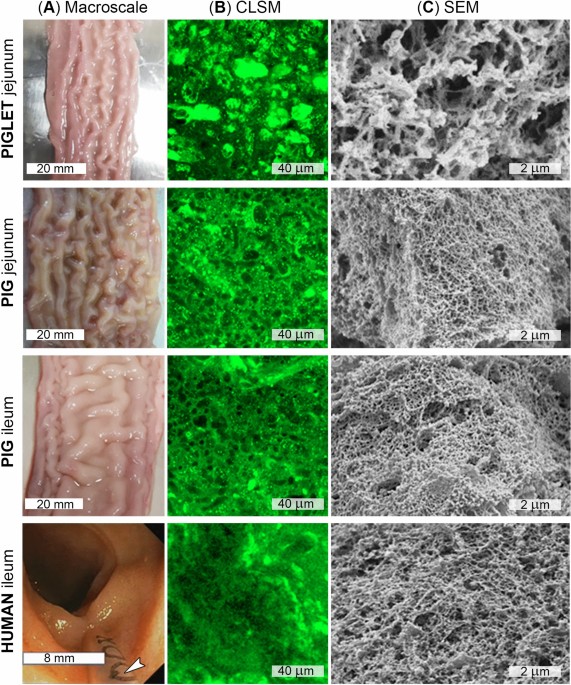
Comparing the permeability of human and porcine small intestinal mucus for particle transport studies | Scientific Reports
Posting Komentar untuk "peyer's patches are found in the distal portion of the"